Hydrogen 101
Hydrogen:
A Flexible Molecule

The Basics of Hydrogen
Did you know?
The sun is essentially a giant
ball of hydrogen gas under-
going fusion into helium.

found in greatest quantities
in water
Hydrogen: An Effective Energy Carrier
Demand for Hydrogen
Hydrogen Production
Electrolysis
To use hydrogen, it must be separated from other substances, like water. There are multiple ways to separate hydrogen from other compounds, but electrolysis is rapidly becoming the preferred choice for large-scale industrial hydrogen production.
Electrolysis is the process of using electricity to split water into hydrogen and oxygen. The reaction takes place in a machine called an electrolyzer. Electrolyzers can range from big to small, and can be suited to support small or large-scale hydrogen production. The electricity required for electrolysis can be tied directly to a non-renewable or renewable source of energy. 10 liters of deionized water is required to produce 1kg of H2 through the electrolysis process.
Hydrogen from Zero Carbon Power
Green hydrogen is generated when a zero carbon energy source like solar or wind is used to power the process of electrolysis. This avoids the burning or use of fossil fuels and prevents greenhouse gas (GHG) emissions from polluting the atmosphere.
Currently, hydrogen is mostly produced from fossil fuels. According to the International Energy Agency (IEA), hydrogen production from fossil fuels has resulted in close to 900 million metric tons (Mt) of CO2 emissions per year, which is about 2.9% of global CO2 emissions. Not only can green hydrogen decarbonize the 2.9% of global CO2 emissions from fossil fuel hydrogen production, but also play a role in decarbonizing the broader industrial, transportation and power sectors – which combined contribute roughly 86% of global CO2 emissions.

On-site vs. Off-site Hydrogen Production
Did you know…
Ammonia is an effective hydrogen carrier and can be used in fuel cells, turbines or engines. Green ammonia is produced by combining hydrogen from water with nitrogen from the air. Due to its high hydrogen content (17.65 wt%) and ability to be liquified at -33°C, ammonia is increasingly recognized as a clean fuel.
Fuel Cells
Fuel cells generate electricity through an electrochemical reaction. In a fuel cell, hydrogen and oxygen are combined to generate electricity, and the only byproducts are heat and water. The water can be used for other industrial operations or reused in the electrolysis process.
Fuel cells that use green hydrogen are 100% clean, meaning they produce zero carbon emissions. Fuel cells do not need to be recharged like batteries; as long as there is a constant source of fuel, they continue to
produce electricity.
Green Hydrogen - A Flexible Molecule
Green hydrogen is an important piece of the decarbonization puzzle for multiple sectors, for example, chemical industries, steel manufacturers, power sector, aviation, maritime, and road transport.
- Hydrogen and derivative e-fuels like ammonia or methanol can be used as fuel for transportation.
- Hydrogen is stored and used for stationary power, building heat, and industrial and manufacturing sectors.
- Fuel cells can provide non-stop power for critical load functions, such as data centers, telecommunication towers, and emergency response systems.
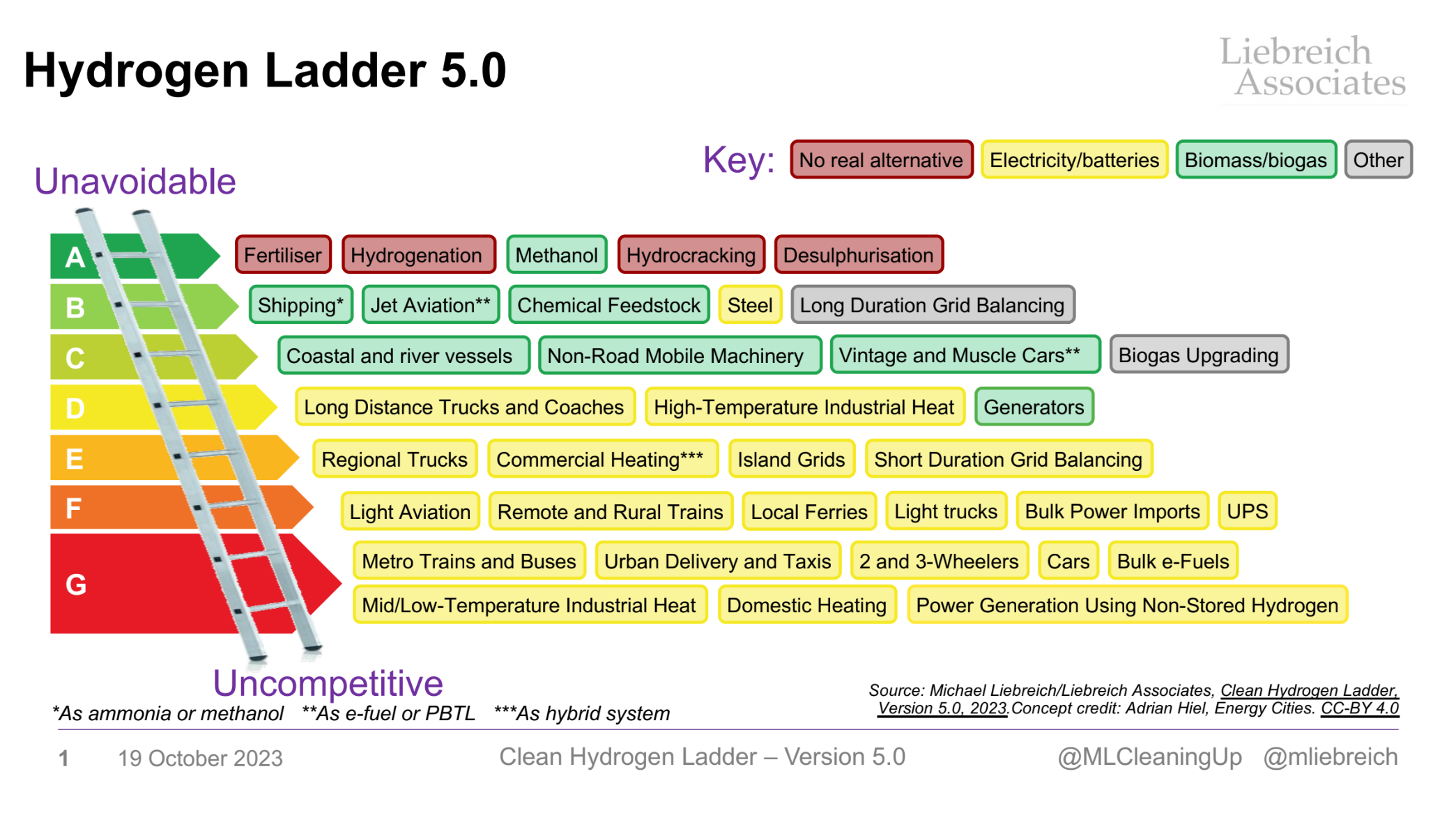
This ladder, developed by Liebreich Associates, provides the current view on sectors where green H2 can prove to be a competitive, greener fuel, and sectors in which it is yet to penetrate or be competitive. It is important to note that most of the alternate technologies mentioned are also undergoing rapid disruptions and technological advancement. All these technologies will continue to co-exist and thrive in a decarbonized society. Multiple factors govern the pace of green H2 adoption across these sectors such as innovation, cost competitiveness, and policy. We expect the uncompetitive sectors’ ladder rungs to shrink with time as green H2 continues to reduce in cost through innovation and policy mechanisms.
Want to learn more about hydrogen?
The U.S. Department of Energy’s (DOE) Hydrogen and Fuel Cell Technologies Office (HFTO)
The U.S. Department of Energy’s (DOE) Energy Earthshots Initiative
National Renewable Energy Laboratory’s (NREL) Hydrogen and Fuel Cell Research & Development
International Energy Association’s (IEA) Research on Hydrogen
